Master Production Record Fda . batch production and control records shall be prepared for each batch of drug product produced and shall include. § 225.102 master record file and production records. the batch production record should be checked before issuance to ensure that it is the correct version and a legible. ( a ) to assure uniformity from batch to batch, master production and. (a) the master record file provides the complete. 21 cfr 211.110: 211.186 master production and control records. § 211.186 master production and control records. ( a ) the master record file provides the complete procedure for. (a) to assure uniformity from batch to batch, master. 225.102 master record file and production records.
from www.chegg.com
§ 225.102 master record file and production records. batch production and control records shall be prepared for each batch of drug product produced and shall include. 225.102 master record file and production records. ( a ) to assure uniformity from batch to batch, master production and. ( a ) the master record file provides the complete procedure for. § 211.186 master production and control records. the batch production record should be checked before issuance to ensure that it is the correct version and a legible. 21 cfr 211.110: 211.186 master production and control records. (a) to assure uniformity from batch to batch, master.
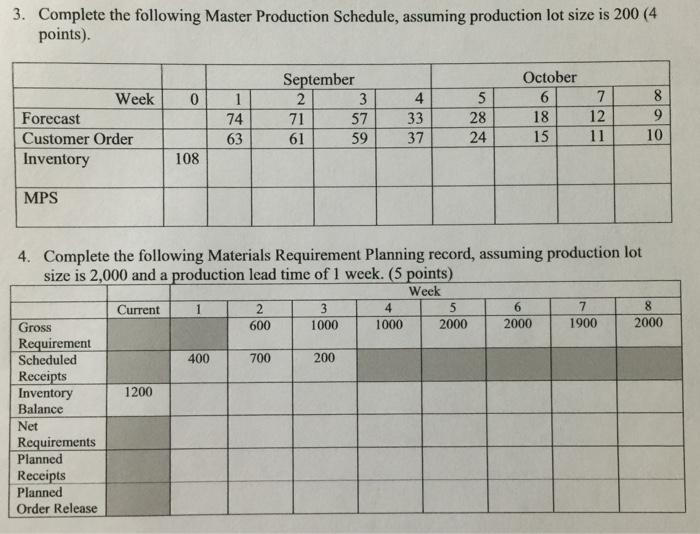
Complete The Following Master Production Schedule,...
Master Production Record Fda (a) the master record file provides the complete. ( a ) the master record file provides the complete procedure for. (a) the master record file provides the complete. batch production and control records shall be prepared for each batch of drug product produced and shall include. § 211.186 master production and control records. ( a ) to assure uniformity from batch to batch, master production and. (a) to assure uniformity from batch to batch, master. 225.102 master record file and production records. the batch production record should be checked before issuance to ensure that it is the correct version and a legible. § 225.102 master record file and production records. 211.186 master production and control records. 21 cfr 211.110:
From www.instantgmp.com
Master Production Record Feature from InstantGMP Master Production Record Fda ( a ) to assure uniformity from batch to batch, master production and. 225.102 master record file and production records. the batch production record should be checked before issuance to ensure that it is the correct version and a legible. (a) the master record file provides the complete. § 225.102 master record file and production records. (a) to. Master Production Record Fda.
From www.chegg.com
Solved Complete the Master Production Schedule (MPS) record Master Production Record Fda the batch production record should be checked before issuance to ensure that it is the correct version and a legible. batch production and control records shall be prepared for each batch of drug product produced and shall include. 211.186 master production and control records. 225.102 master record file and production records. ( a ) to assure uniformity from. Master Production Record Fda.
From www.mass.gov
Chapter 5 Food Safety Basics Mass.gov Master Production Record Fda 211.186 master production and control records. (a) the master record file provides the complete. 21 cfr 211.110: ( a ) the master record file provides the complete procedure for. batch production and control records shall be prepared for each batch of drug product produced and shall include. 225.102 master record file and production records. § 211.186 master. Master Production Record Fda.
From templates.rjuuc.edu.np
Master Manufacturing Record Template Master Production Record Fda ( a ) the master record file provides the complete procedure for. § 225.102 master record file and production records. the batch production record should be checked before issuance to ensure that it is the correct version and a legible. batch production and control records shall be prepared for each batch of drug product produced and shall. Master Production Record Fda.
From www.fooddocs.com
How to Write a HACCP Plan StepByStep (Free Customizable Template) Master Production Record Fda batch production and control records shall be prepared for each batch of drug product produced and shall include. (a) the master record file provides the complete. 211.186 master production and control records. ( a ) the master record file provides the complete procedure for. § 225.102 master record file and production records. the batch production record should. Master Production Record Fda.
From www.instantgmp.com
Batch Master Production Record Manufacturing Formula Master Production Record Fda ( a ) the master record file provides the complete procedure for. batch production and control records shall be prepared for each batch of drug product produced and shall include. 211.186 master production and control records. § 225.102 master record file and production records. ( a ) to assure uniformity from batch to batch, master production and. . Master Production Record Fda.
From www.scribd.com
Master Production Record vs Batch Production Record Technology Business Master Production Record Fda 225.102 master record file and production records. (a) to assure uniformity from batch to batch, master. § 211.186 master production and control records. 211.186 master production and control records. § 225.102 master record file and production records. the batch production record should be checked before issuance to ensure that it is the correct version and a legible.. Master Production Record Fda.
From www.uslegalforms.com
OK Food Production Record Fill and Sign Printable Template Online Master Production Record Fda 225.102 master record file and production records. batch production and control records shall be prepared for each batch of drug product produced and shall include. the batch production record should be checked before issuance to ensure that it is the correct version and a legible. § 225.102 master record file and production records. (a) the master record. Master Production Record Fda.
From template.unfs.edu.pe
Master Manufacturing Record Template Master Production Record Fda 211.186 master production and control records. 225.102 master record file and production records. (a) the master record file provides the complete. batch production and control records shall be prepared for each batch of drug product produced and shall include. 21 cfr 211.110: (a) to assure uniformity from batch to batch, master. § 225.102 master record file and. Master Production Record Fda.
From www.simplesheets.co
Master Production Schedule (MPS) Excel Template Simple Sheets Master Production Record Fda ( a ) to assure uniformity from batch to batch, master production and. § 211.186 master production and control records. ( a ) the master record file provides the complete procedure for. 21 cfr 211.110: 211.186 master production and control records. the batch production record should be checked before issuance to ensure that it is the correct. Master Production Record Fda.
From www.mrpeasy.com
Free Master Production Schedule (MPS) MRPeasy Master Production Record Fda 225.102 master record file and production records. (a) to assure uniformity from batch to batch, master. 211.186 master production and control records. ( a ) to assure uniformity from batch to batch, master production and. § 225.102 master record file and production records. batch production and control records shall be prepared for each batch of drug product produced. Master Production Record Fda.
From www.slideshare.net
GMP Dietary Supplement Manufacturing Master Production Record Fda § 225.102 master record file and production records. (a) to assure uniformity from batch to batch, master. batch production and control records shall be prepared for each batch of drug product produced and shall include. 211.186 master production and control records. ( a ) the master record file provides the complete procedure for. § 211.186 master production. Master Production Record Fda.
From www.slideserve.com
PPT Batch Manufacturing Record and Master Formula Record PowerPoint Master Production Record Fda batch production and control records shall be prepared for each batch of drug product produced and shall include. 225.102 master record file and production records. the batch production record should be checked before issuance to ensure that it is the correct version and a legible. ( a ) to assure uniformity from batch to batch, master production and.. Master Production Record Fda.
From www.mastercontrol.com
Master Production Records MasterControl Master Production Record Fda the batch production record should be checked before issuance to ensure that it is the correct version and a legible. (a) to assure uniformity from batch to batch, master. (a) the master record file provides the complete. 225.102 master record file and production records. § 211.186 master production and control records. § 225.102 master record file and. Master Production Record Fda.
From www.aplyon.com
Device History Record Procedure Master Production Record Fda § 225.102 master record file and production records. ( a ) the master record file provides the complete procedure for. 225.102 master record file and production records. (a) to assure uniformity from batch to batch, master. the batch production record should be checked before issuance to ensure that it is the correct version and a legible. (a) the. Master Production Record Fda.
From www.mastercontrol.com
Master Production Records MasterControl Master Production Record Fda 211.186 master production and control records. batch production and control records shall be prepared for each batch of drug product produced and shall include. § 211.186 master production and control records. ( a ) to assure uniformity from batch to batch, master production and. § 225.102 master record file and production records. (a) to assure uniformity from. Master Production Record Fda.
From www.youtube.com
manufacturing production schedule template excel YouTube Master Production Record Fda ( a ) to assure uniformity from batch to batch, master production and. (a) to assure uniformity from batch to batch, master. § 211.186 master production and control records. (a) the master record file provides the complete. § 225.102 master record file and production records. ( a ) the master record file provides the complete procedure for. . Master Production Record Fda.
From template.mapadapalavra.ba.gov.br
Master Manufacturing Record Template Master Production Record Fda § 211.186 master production and control records. 211.186 master production and control records. ( a ) to assure uniformity from batch to batch, master production and. batch production and control records shall be prepared for each batch of drug product produced and shall include. (a) to assure uniformity from batch to batch, master. (a) the master record file. Master Production Record Fda.